![]()
The Phenomenon of Fluorescence
The
basic principle of fluorescence was known by the middle of the 19th
century. It was Stokes who observed that the mineral fluoresces
when ultraviolet light is directed upon it; he coined the word "fluorescence".
Stokes
observed that the fluorescing light is in longer wavelengths than
those of the excitation light wavelengths and the fluorescence emission
sustains only during the absorption of the excitation light. Below
figure illustrates the process of fluorescence.
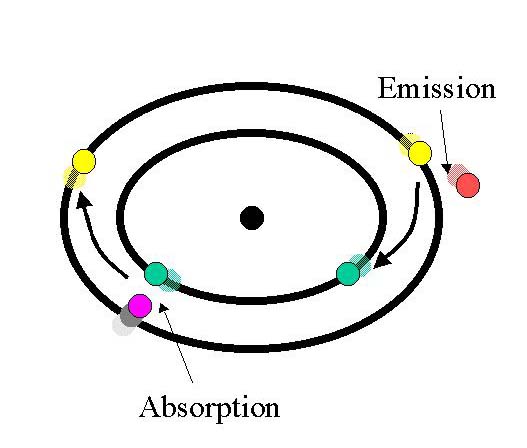
The
process of fluorescence is followed;
A photon of external radiation
(purple) collides with an electron in the atom (green), exciting and elevating it to a higher
energy level(yellow). Subsequently, the excited electron (yellow) relaxes to a lower level (green) and emits light in the form of a lower-energy photon
(red).